COPDGene was initially funded by NIH because of interest in the genetic origins of the disease. The study used contemporary definitions of disease and exposure to build the cohort – all participants had a > 10 pack year history of cigarette smoking and met other criteria for inclusion. The primary analysis was a Genome Wide Association study (GWAS) where the study intended to compare people with and without COPD as defined by the GOLD criteria;
Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria
Classification of severity of airflow limitation in COPD:
In pulmonary function testing, a postbronchodilator FEV1/FVC ratio of <0.7 is commonly considered diagnostic for COPD. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) system categorizes airflow limitation into stages. In patients with FEV1/FVC <0.7:
- GOLD 1 – mild: FEV1 ≥80% predicted
- GOLD 2 – moderate: 50% ≤ FEV1 <80% predicted
- GOLD 3 – severe: 30% ≤ FEV1 <50% predicted
- GOLD 4 – very severe: FEV1 <30% predicted.
From the start of recruitment, the study investigators noticed a few aspects of the population that didn’t exactly match what they expected. Smokers with FEV1/FVC >0.7 but FEV1% < 80% were common enough that the study felt that it was not ethical to leave them out of the recruiting and process. Also people who did not meet GOLD criteria expressed symptoms that indicated a problem with their lungs that was quite similar to COPD and there were participants who were already treated with drugs that are typically prescribed for COPD. These observations and changes to the original protocol were based on epidemiologic observations and they have led to COPDGene making unique contributions to the understanding of COPD across the world. Five unique contributions stand out;
Early COPD
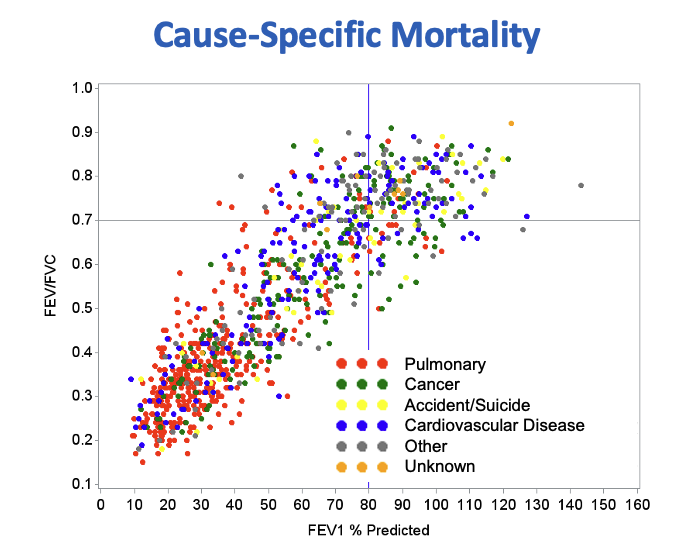
COPDGene has identified aspects of COPD that occur before the loss of pulmonary function identifies the disease. We observed and reported on COPD treatment practices that are reasonably common in clinical practice where symptoms and a history of exposures are used by clinicians to establish a diagnosis without performing spirometry, these participants are then treated with COPD medications though the outcomes of that treatment do not appear to halt the progression of disease. Early COPD as a concept has been investigated by several groups within COPDGene using genetics, -omics and other statistical approaches that identify participants who subsequently show progressive loss of pulmonary function and continued lung damage visible on imaging over time.
Early COPD likely contributes to early mortality related to both this early manifestation of disease and to cigarette smoking in general. COPDGene has shown pulmonary associated mortality in participant who do not meet current criteria for COPD in terms of the ratio of FEV1/FVC.
Subtypes of COPD
Alongside the observation that Early COPD is present in smokers who haven’t lost pulmonary function in a pattern that is commonly used to diagnose the disease, COPDGene has shown that there are subtypes of COPD that were hinted at in the past but are identifiable in our study. Primary among subtypes of COPD are people who develop early emphysema characterized by destruction of lung tissue and loss of pulmonary function. The study has shown that this pattern is common but can lead to a fairly stable phenotype where people lose some lung function and then stabilize for, in some cases 10 years of follow up by the study. This subtype is characterized by loss of FEV1/FVC ratio initially, increase in emphysema and a slow progression. The other primary subtype is characterized by increased evidence of inflammation in the lungs especially in the airways where we can observe increased thickening of the airway walls, increased biomarkers of inflammation and other indications of systemic inflammation. People with this pattern tend to lose both FEV1 and FVC simultaneously but their ratio doesn’t change until later in the course of the disease.
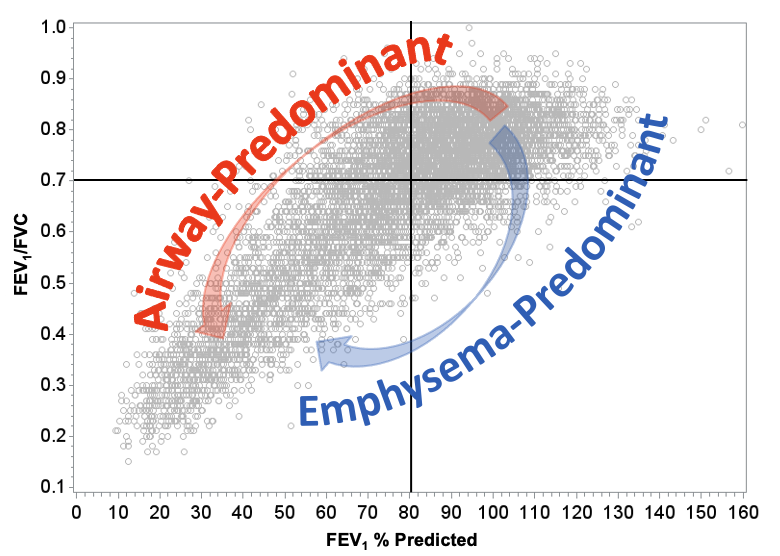
COPDGene identified these participants because the study modified its design early in the process of recruitment to include people who were typically excluded from other studies of COPD. Having identified this new group of people at high risk for continued disease progression and also mortality events, the study named this group Preserved Ratio, Impaired Spirometry (PRISm). Other approaches to identifying subtypes showed that this airway predominant group was present most frequently in PRISm but were also present in people with FEV1/FVC < 0.7 suggesting that progression of disease occurred through a pathway that hadn’t been observed in past studies.
Other approaches to subtyping COPD have also generated innovative and unique observations, including genetic risk scores, statistical clustering approaches and statistical approaches using machine learning techniques that combing thousands of variables generated by genetics and -omics data that have identified common and rare groups of people who are at risk for pulmonary function loss and other clinically important outcomes outside of the classical definition of COPD.
How progressive loss of pulmonary function occurs in COPD
COPD has long been known to be a chronic condition that can progress slowly making it very difficult to use short term study design to investigate people who are at risk of losing large amounts of pulmonary function, require oxygen supplementation and other forms of support. COPDGene has followed its participants over 15 years using three study visits with a fourth visit beginning now which has allowed us to observe people making this transition from early to late stages of COPD.
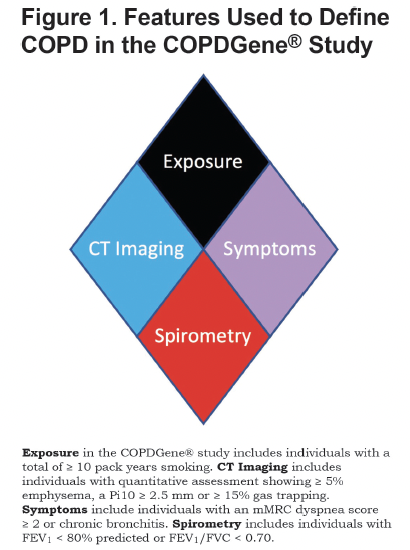
New criteria for diagnosis of COPD
Years of follow up data, extensive phenotyping of participants and hundreds of coinvestigators providing unique insight into COPDGene data have allowed us to build new criteria for diagnosing COPD. COPD2019 was an initial effort by the study to inform the pulmonary community of how our observations could translate into clinical practice by expanding the definition of COPD from a spirometricly defined disease and into one that considers for exposures, symptoms, CT evidence of lung damage and also spirometry. COPDGene isn’t unique in attempting to shift the understanding of this disease but our continued efforts have resulted in a broader discussion which we continue to participate in.
Factors that may optimize drug trials aimed at identifying new medications to use for the treatment of COPD
COPDGene has begun to translate its observations into recommendations for strategies that will generate better drug trial designs that incorporate Early COPD, COPD Subtypes, risk factors for progression to severe COPD and newly diagnosed COPD using newly identified criteria for the disease.