The COPDGene Study uses CT imaging (with relatively low radiation dose) to help us accurately visualize various forms of lung and airway diseases associated with COPD. Along with spirometry and genetic SNPs that may be associated with COPD, the use of longitudinal CT data has allowed for the re-characterization of COPD phenotypes, the assessment of morphologic changes in COPD over time, and the development of a more complete picture of COPD. CT scanning has been pivotal in evaluation of smoking related lung diseases, resulting in more than 200 imaging-related manuscripts to date.
CT Scan findings in cigarette smokers
Cigarette smokers with and without COPD may have a wide spectrum of pulmonary abnormalities on CT imaging. The main findings seen on CT are emphysema, bronchitis and bronchiolitis. Based on the data provided from the COPDGene Study, we now recognize that imaging of smoking-related lung disease varies greatly between individuals who may have similar physiologic impairment. For instance, some patients with severe COPD may have predominantly emphysema on CT, others may have findings of large and small airways disease with little or no emphysema, and others may have a mix. These differences can help to guide therapy as those with airway disease predominant COPD may benefit from different treatment options from those with emphysema predominant COPD.
Emphysema. Emphysema occurs when there is permanent enlargement of distal airspaces with associated destruction of the walls of the alveoli, which are the tiny air sacs in the lungs where oxygen exchange occurs. On CT, emphysema can be directly visualized as focal or confluent areas of low attenuation (darker areas due to increased air), often most evident in the upper lobes.
This coronal CT image in a 48-year-old woman with a 47-pack-year smoking history shows upper lobe predominant emphysema (asterisks). Bronchial wall thickening is also present (white arrows).

Bronchitis In addition to damage to the alveolar walls, inflammation of the large airways (bronchitis), may also occur and manifest as thickening of the airway walls.
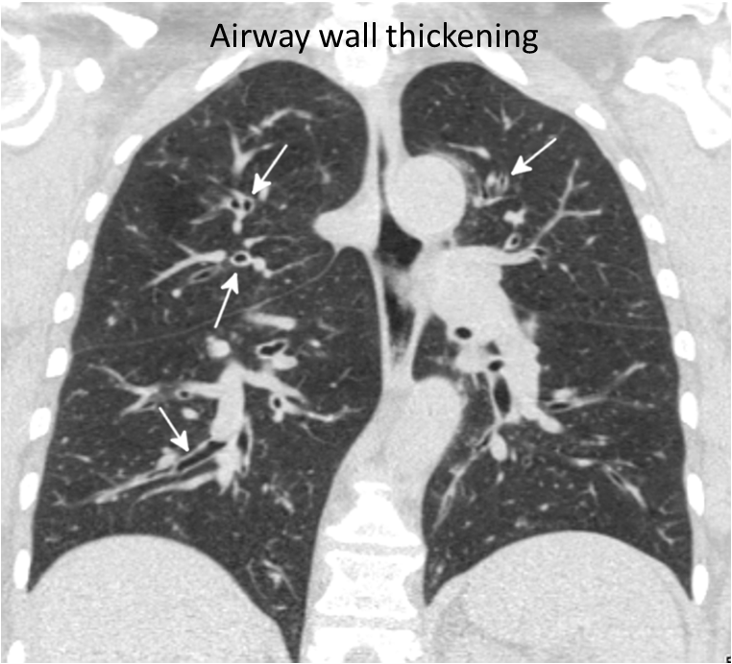
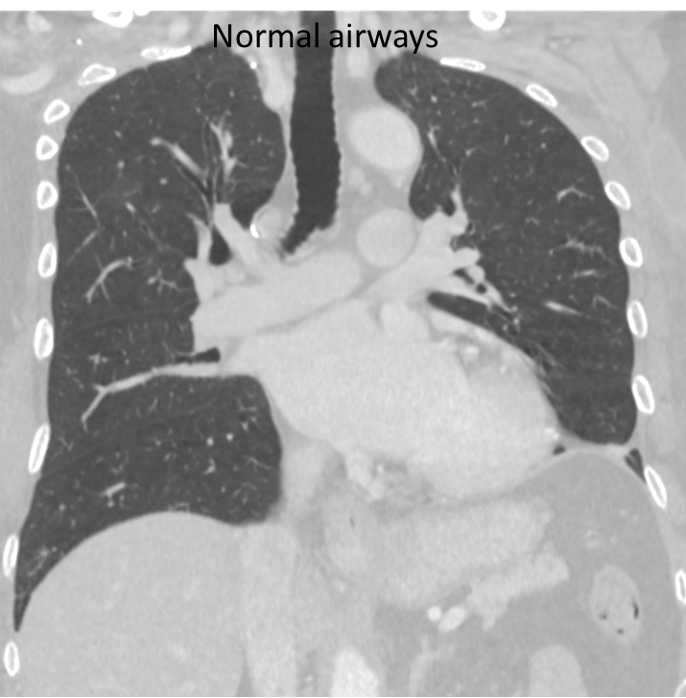
Coronal CT image through the lungs (above left) in a 59-year-old man with a 20-pack-year smoking history shows diffuse thickening of the large airway walls (arrows). For comparison, normal airways in a non-smoker are shown on the right
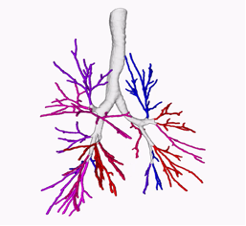
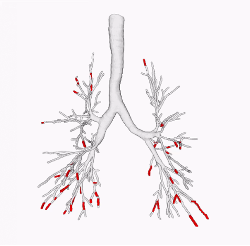
Mucus plugging
In some instances, the airway wall thickening of bronchitis is associated with mucus plugging, which can partially or totally obstruct an airway. Newer research from COPDGene has shown that the presence of these mucus plugs is associated with increased risk of COPD exacerbations, pneumonia, and death.
Sagittal CT image through the right lung in the same person as above shows multiple mucus plugs (arrows) within the thickwalled airways

Bronchiolitis Bronchiolitis refers to injury to the small airways of the lung, which may be obstructive or inflammatory.
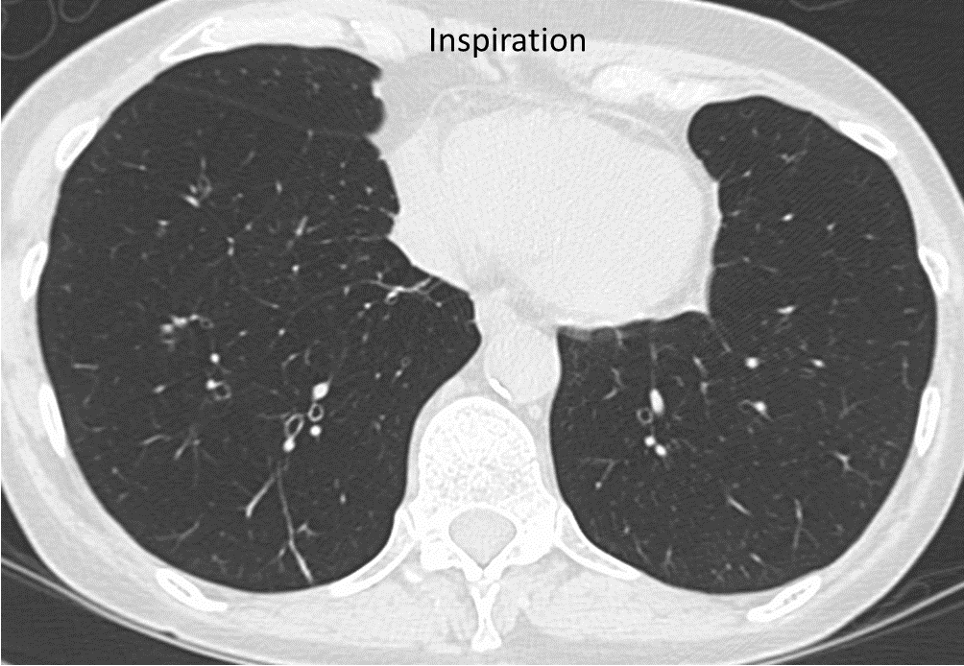
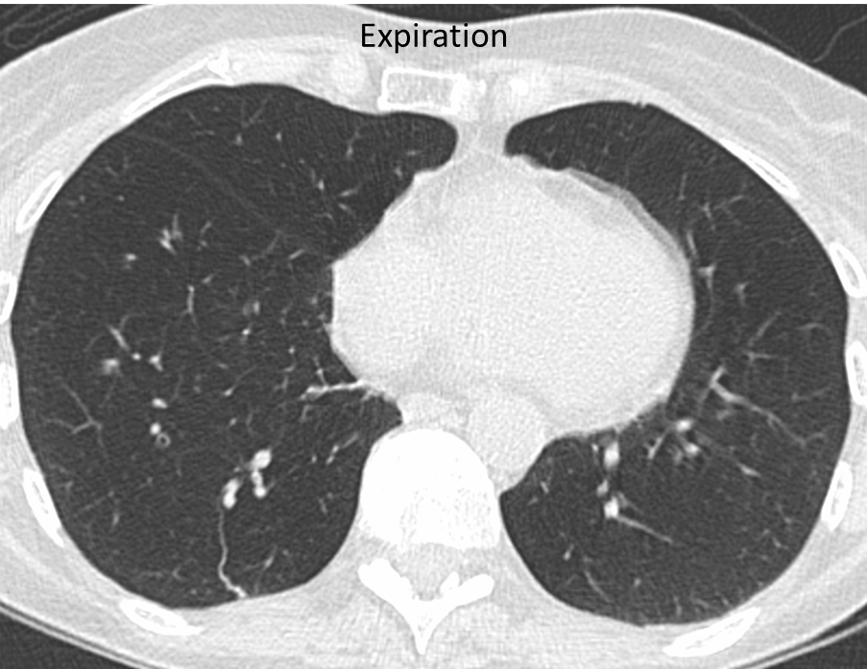
In obstructive bronchiolitis, the lungs fail to empty normally when the person breathes out. This form of bronchiolitis may precede the development of emphysema. It is detected in COPDGene by scanning of the lungs after the person has blown all the air they can out of their lungs (expiratory CT).
In this person with COPD (above), the lungs remain dark on expiration, indicating that air is trapped in the lung by obstructive bronchiolitis.
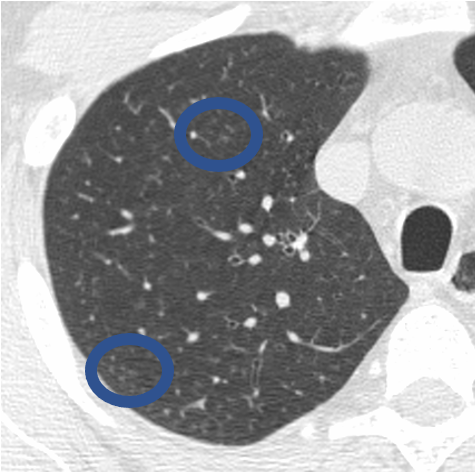
In inflammatory bronchiolitis (also called respiratory bronchiolitis), inflammatory cells surround the small airways, and are seen as clustered small nodules (circled on this image of a person with a heavy smoking history)
Interstitial lung abnormalities While the main components of COPD remain emphysema, bronchitis, and bronchiolitis, the COPDGene Study has also shown that smoking is associated with the presence of previously unsuspected interstitial lung abnormalities (ILA) on CT. ILA are seen in approximately 8% of patients in the COPDGene Study, may be associated with significant symptoms, and may progress over time.
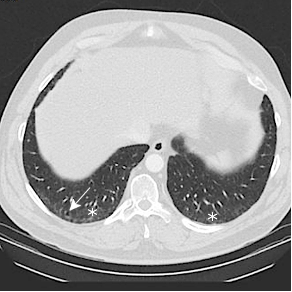
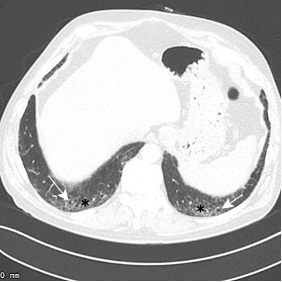
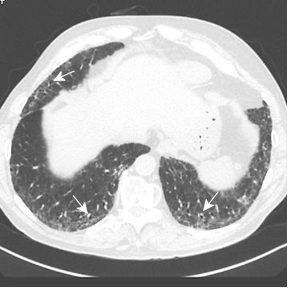
Baseline CT through the lung bases in a 58-year-old man shows subtle subpleural ground glass opacity (white asterisks) with a dilated right lower lobe distal airway (traction bronchiolectasis). CT 5 years later shows progression of subpleural ground glass opacity with a lacy network of fibrosis (reticulation) (black asterisks), and dilated small airways (traction bronchiolectasis) indicating fibrosis. CT 10 years later shows significant progression, representing a probable usual interstitial pneumonia (UIP) pattern of pulmonary fibrosis.
Other findings
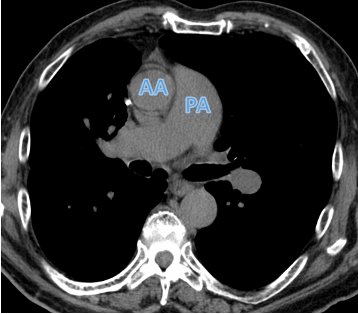
CT can show enlargement of the pulmonary arteries, suggesting increased blood pressure in the pulmonary artery (pulmonary hypertension).
In this individual with COPD, the pulmonary artery (PA) is larger than the adjacent ascending aorta (AA), indicating pulmonary hypertension.
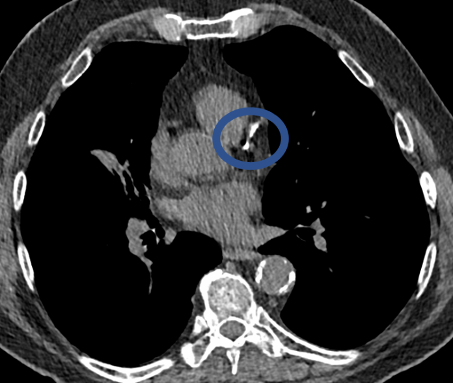
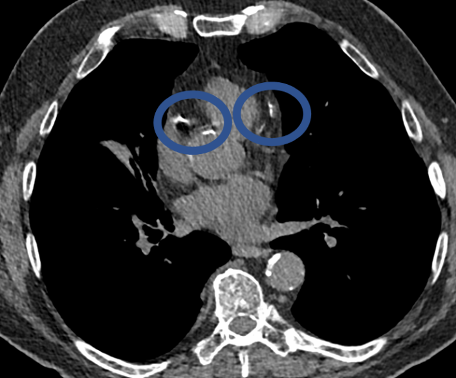
CT can show the presence of calcium in the coronary arteries of the heart (circled in images below). This finding is a predictor of future major adverse cardiac events such as heart attacks and death. Studies from COPDGene have shown that the calcium score obtained using non-ECG-gated CT scans correlates well with ECG-gated studies. Even if a score is not provided, qualitative scoring using categories such as absent, mild, moderate, and severe calcification can help guide future therapy. Lastly, the CT scans in the COPDGene Study can also assess for muscle wasting, increased deposition of body fat, and decrease in bone density in the thoracic spine. Decreased bone density has been found in almost 60% of COPDGene subjects, and was particularly notable in male cigarette smokers.
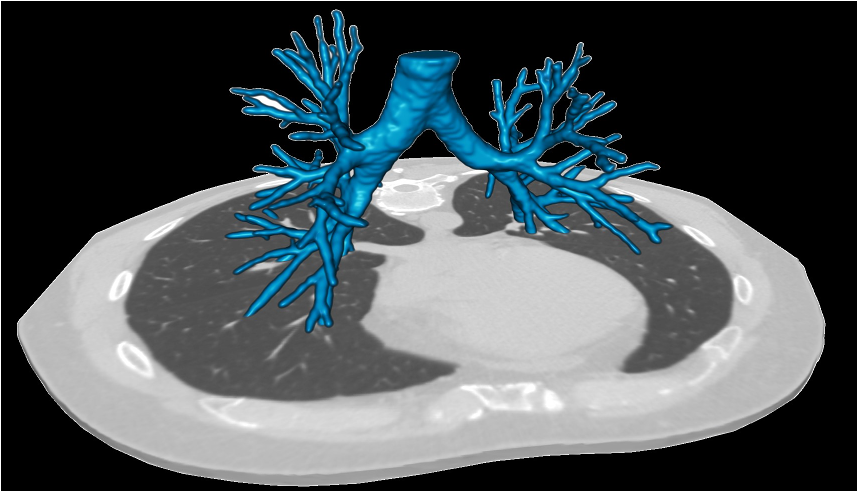
Quantitative Imaging and Artificial Intelligence
While the concordance between radiologists for the assessment of emphysema, bronchial wall thickening, and air trapping is good, it relies on visual, qualitative measurements which can be subjective. For instance, one reader may classify the amount of emphysema as severe, while another may classify it as moderate. Over the past 10-20 years, quantitative analysis of CT scans has been performed using various computer programs. Many methods are based on measurements of lung attenuation. For instance, emphysema is quantified as the percentage of CT lung voxels with attenuation of -950 Hounsfield Units (HU) or less. This approach is fairly straightforward, but it does not provide any information regarding subtypes of emphysema and cannot differentiate low density abnormalities (e.g. air-filled cyst in the lung versus an area of emphysema). Similarly, extent of air trapping on expiratory CT scan is measured as voxels that are less than or equal to -856HU. However, most programs cannot differentiate between areas of emphysema and air trapping on an expiratory scan.
Over the past few years, artificial intelligence (AI) programs have begun to revolutionize how quantitative imaging is performed in radiology. Researchers in the COPDGene Study have successfully applied deep learning algorithms to automate, to a high degree of precision, the numerous quantitative metrics that can be derived from a single study. AI algorithms used in COPDGene have been able to differentiate between subcategories of emphysema, identify early evidence of fibrosis, automatically trace the bronchi and pulmonary vessels to assess for morphologic changes, accurately measure air trapping while excluding areas of increased image noise or emphysema, automatically calculate the main PA to aorta ratio, the right ventricle to left ventricle ratio, and coronary artery calcium score, measure the amount of skeletal muscle and thoracic fat, and measure bone density. This is just a small number of applications of AI being studied, all with the hopes of improving the accuracy of radiologic assessments of smoking-related lung disease.

Radiation Risk from CT
CT scans are acquired using ionizing radiation. Exposure to ionizing radiation may cause some increase in risk of cancer. The precise level of risk is not known, but appears relatively small. Nonetheless, it is important to minimize any risk. Because CT may be associated with a small increased risk of cancer, CT should be performed only where this risk is outweighed by potential clinical benefit, or in the context of an approved research study. The CT scans should be performed with the lowest possible radiation exposure. The average amount of radiation exposure during the chest CT scan in COPDGene is approximately 3.5 mSv (mSv stands for milliSievert, which is a measure of the dose of low levels of radiation.) The average amount of background radiation that the general population is exposed to in the United States is 3 mSv per year. Thus, the average amount of radiation from the CT scan is equivalent to about one and one third years of normal background radiation.
Key references from COPDGene imaging research:
- Lynch DA, Moore CM, Wilson CS, Nevrekar D, Jennerman T, Humphries SM, Austin JHM, Grenier PA, Kauczor H, Han MK, Regan EA, Make BJ, Bowler RP, Beaty TH, Curan-Everett D, Hokanson JE, Curtis JL, Silverman EK, Crapo JD, COPDGene Investigators. CT based visual classification of emphysema: Association with mortality in the COPDGene study. Radiology 2018 Sep; 288(3):859-866. PMID: 29762095 PMCid: 6122195
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6122195/ - Ash SY, San José Estépar R, Fain SB, Tal-Singer R, Stockley RA, Nordenmark LH, Rennard S, Han MK, Merrill D, Humphries SM, Diaz AA, Mason SE, Rahaghi FN, Pistenmaa CL, Sciurba FC, Vegas-Sánchez-Ferrero G, Lynch DA, Washko GR; COPDGene Investigators and the COPD Biomarker Qualification Consortium. Relationship between emphysema progression at CT and mortality in ever-smokers: results from the COPDGene and ECLIPSE cohorts. Radiology 2021 Apr; 299(1):222-231. PMID: 33591891 PMCid: 7997617
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7997617/ - Maselli DJ, Yen A, Wang W, Okajima Y, Dolliver WR, Mercugliano C, Anzueto A, Restrepo MI, Aksamit TR, Basavaraj A, Aliberti S, Young KA, Kinney GL, Wells JM, San José Estépar R, Lynch DA, Diaz AA. Small Airway Disease and Emphysema Are Associated with Future Exacerbations in Smokers with CT-derived Bronchiectasis and COPD: Results from the COPDGene Cohort. Radiology 2021 Sep; 300(3):706-714. PMID: 34156303 PMCid: 8409101
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8409101 - Wade RC, Simmons JP, Boueiz A, Gregory A, Wan ES, Regan EA, Bhatt SP, Han MK, Bowler RP, Crapo JD, Silverman EK, Washko GR, Dransfield MT, Wells JM. Pulmonary artery enlargement is associated with exacerbations and mortality in ever-smokers with Preserved Ratio Impaired Spirometry (PRISm). Am J Respir Crit Care Med 2021 Aug 15; 204(4):481-485. PMID: 34014810 PMCid: 8480250
https://pubmed.ncbi.nlm.nih.gov/34014810/ - Hata A, Hino T, Putman RK, Yanagawa M, Hida T, Menon AA, Honda O, Yamada Y, Nishino M, Araki T, Valtchinov VI, Jinzaki M, Honda H, Ishigami K, Johkoh T, Tomiyama N, Christiani DC, Lynch DA, San José Estépar R, Washko GR, Cho MH, Silverman EK, Hunninghake GM, Hatabu H; COPDGene Investigators. Traction Bronchiectasis/Bronchiolectasis on CT Scans in Relationship to Clinical Outcomes and Mortality: The COPDGene Study. Radiology 2022 Sep; 304(3):694-701. PMID: 35638925 PMCid: 9434811
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9434811/ - Rose JA, Menon AA, Hino T, Hata A, Nishino M, Lynch DA, Rosas IO, El-Chemaly S, Raby BA, Ash SY, Choi B, Washko GR, Silverman EK, Cho MH, Hatabu H, Putman RK, Hunninghake GM. Suspected Interstitial Lung Disease in COPDGene. Am J Respir Crit Care Med 2023 Jan 1; 207(1):60-68. PMID: 35930450 PMCid: 9952869
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9952869/ - Baraghoshi D, Strand M, Humphries SM, San José Estépar R, Vegas Sanchez-Ferrero G, Charbonnier JP, Latisenko R, Silverman EK, Crapo JD, Lynch DA. Quantitative CT evaluation of emphysema progression over 10 years in the COPDGene Study. Radiology 2023 May; 307(4):e222786. PMID: 37039685 PMCid: 10286952
https://pubmed.ncbi.nlm.nih.gov/37039685/ - Diaz AA, Orejas JL, Grumley S, Nath HP, Wang W, Dolliver WR, Yen A, Kligerman SJ, Jacobs K, Manapragada PP, Abozeed M, Aziz MU, Zahid M, Ahmed AN, Terry NL, San José Estépar R, Kim V, Make BJ, Han MK, Sonavane S, Washko GR, Cho M, San José Estépar R. Airway-Occluding Mucus Plugs and Mortality in Patients With Chronic Obstructive Pulmonary Disease. JAMA 2023 Jun 6; 329(21):1832-1839. PMID: 37210745 PMCid: 10201404
https://pubmed.ncbi.nlm.nih.gov/37210745/ - Diaz AA, Wang W, Orejas JL, Elalami R, Dolliver WR, Nardelli P, San José Estépar R, Choi B, Pistenmaa CL, Ross JC, Maselli DJ, Yen A, Young KA, Kinney GL, Cho MH, San José Estépar R. Suspected bronchiectasis and mortality in adults with a history of smoking who have normal and impaired lung function: A cohort study. Ann Intern Med 2023 Oct; 176(10):1340-1348. PMID: 37782931 PMCid: 10809158
https://pubmed.ncbi.nlm.nih.gov/37782931/ - Ash SY, Choi B, Oh A, Lynch DA, Humphries SM; COPDGene Study Investigators. Deep Learning Assessment of Progression of Emphysema and Fibrotic Interstitial Lung Abnormality. Am J Respir Crit Care Med 2023 Sept 15; 208(6):666-675. PMID: 37364281 PMCid: 10515569
https://pubmed.ncbi.nlm.nih.gov/37364281/ - Mettler SK, Nath HP, Grumley S, Orejas JL, Dolliver WR, Nardelli P, Yen AA, Kligerman SJ, Jacobs K, Manapragada PP, Abozeed M, Aziz MU, Zahid M, Ahmed AN, Terry NL, Elalami R, San José Estépar R, Sonavane S, Billatos E, Wang W, San José Estépar R, Richards JB, Cho MH, Diaz AA. Silent airway mucus plugs in COPD and clinical implications. Chest 2023 Nov 25:S0012-3692(23)05825-7. doi: 10.1016/j.chest.2023.11.033. Online ahead of print. PMID: 38013161 PMCid:
https://pubmed.ncbi.nlm.nih.gov/38013161/